- (Olea europaea L.) on reactive species scavengers
Patricia Goldschmidt Lins, Silvana Marina Piccoli Pugine, Antonio Márcio Scatolini, Mariza Pires de Melo
Department of Basic Science, Faculty of Animal Science and Food Engineering (FZEA), University of São Paulo, Brazil
Correspondence: Mariza Pires de Melo, Department of Basic Science, Faculty of Animal Science and Food Engineering (FZEA), University of São Paulo, Pirassununga, SP, Brazil, Tel +55-19-35654276
Received: September 18, 2019 | Published: March 20, 2020
Citation: Lins PG, Pugine SMP, Scatolini AM, et al. Antioxidant actions of olive leaf extract (Olea europaea L.) on reactive species scavengers. J Anal Pharm Res.2020;9(2):68-71. DOI: 10.15406/japlr.2020.09.0035
Abstract
This study aimed to evaluate in vitro antioxidant action of olive leaf extract (Olea europaea L.) by: i) Trolox equivalent antioxidant capacity (TEAC) by ABTS•+, DPPH and Ferric reducing antioxidant power (FRAP) assays; ii) scavenging of superoxide anion (O2•-), hypochlorous acid (HOCl) and nitric oxide (NO), compared to ascorbic acid. Results showed TEAC values as 0.148±0.015, 0.215±0.076 and 0.282±0.023 gram of trolox equivalent per gram of dry extract weight, to respective ABTS•+, DPPH• and FRAP. Olive leaf extract was better antioxidant than ascorbic acid on O2•- scavenging, at concentrations over 50 µg/mL; similar effects on NO scavenging for both was seen and on HOCl inhibition, the extract showed lower antioxidant action than ascorbic acid at all concentrations. Olive leaf extract showed potentiality to be used as antioxidant in biological systems.
Keywords: hypochlorous acid, nitric oxide, olive leaves, superoxide anion
Introduction
The olive tree (Olea europaea L.) has great historical and commercial importance, being its main product olive oil, well known for its antioxidant effects and health benefits.1 The olive leaves have shown high bioactive compounds content, including phenolic compounds.2,3 These compounds have important antioxidant potential, mainly due to their structure, ideal for free radical scavenging, in which polyphenols can avoid chain reactions.4,5 Recently, studies have shown that olive leaves have antioxidant activity on induced hemolysis and free radicals with biological characteristics.6 Thus the olive leaf extract has been showing interest due to its potential use in the medical, pharmaceutical, cosmetic and food fields.7–10
Antioxidant activity has been evaluated by several chemical analysis methods.11 Synthetic free radical as DPPH• (2,2-diphenyl-1-picrylhydraza) and ABTS•+[2,2-azino-bis- (3-ethylbenzothiazoline-6-sulfonic acid)] were used for antioxidant activity evaluation.12,13Other methods based on the ability of antioxidants to reduce metal ions such as ferric and cupric has been used, as FRAP (ferric ion reducing power) and CUPRAC (cupric ion reducing capacity) analyzes. In addition, antioxidant activity on reactive oxygen species with biological characteristic has been employed. Reactive species as hypochlorous acid (HOCl), hydrogen peroxide (H2O2), superoxide anion (O2•-), hydroxyl radical (HO•) and nitric oxide (NO) were used.6,11,14
The aim of this study was to evaluate the in vitroantioxidant action of Olea europaea L. leaf extract by: i) trolox equivalent antioxidant activity using three methods as ABTS•+, DPPH• and FRAP; ii) species with biological characteristics as O2•-, HOCl and NO compared to ascorbic acid antioxidant.
Materials and methods
Plants and chemicals
Commercial micronized powdered olive leaves were provided by Folhas de Oliva® (Brazil). All chemical compounds were obtained from Sigma-Aldrich (St. Louis, USA).
Olive leaf extract preparation
Ten grams of dried/micronized olive leaves were subjected to removal of n-hexane-soluble compounds using a Soxhlet extractor.15 The sample was subjected to extraction with 200mL of methanol/water (80:20v/v), under agitation at 170rpm (Shaker TE-420, Tecnal, Brazil), protected from light at 25°C for 24h, and was centrifuged at 1970g for 10min. The supernatant was filtered through Whatman (11 mm) filter paper and methanol was removed under reduced pressure (£50°C) (Rotary evaporator SL-126, Solab, Brazil). The concentrated extract was diluted in water (final volume 200 mL), sonicated (Ultrasonic Disruptor Unique cells, Brazil) (2 cycles of 20s at 100W), and then centrifuged at 1 970 g for 5 min. The supernatant was frozen and freeze-dried (Lyophilizer K-202, Liobras, Brazil) to obtain dried extract. The extract was diluted in phosphate-buffered saline (PBS), pH 7.4. The extract preparation was performed six times.
Determination of total phenolic
The Folin-Ciocalteau colorimetric method was used for estimation of total polyphenol content of the extract.16The extract (0.2 mg/mL) was incubated for 2 min with Folin-Ciocalteau reagent, and then 0.708 mol/L sodium carbonate solution was added. After 1 h of incubation at 25 °C, the absorbance at 760 nm was determined. The results were expressed as gallic acid equivalent (GAE) per gram dry weight (dw).
Antioxidant action on ABTS•+, DPPH• and FRAP
ABTS•+ scavenging activity was determined according to Re et al.,17 with modifications. ABTS•+ radical was obtained from reaction of 7.0mmol/L ABTS with 2.45mmol/L ammonium persulfate in the dark, under constant agitation at room temperature for 12-16h. ABTS•+ solution was diluted in ethanol to obtain an absorbance of approximately 0.70 at 750nm. Final reaction mixtures containing different concentrations of extract (3.3-26.7μg/mL) and ABTS•+ solution were incubated for 6 min in the dark at room temperature and the absorbance determined at 750 nm. Inhibition percentage of ABTS•+ was calculated according to the following equation:
ABTS•+ inhibition (%) = [1- (A/AC0)] x 100
Where, AC0 is the absorbance of the control assay (no extract) at the initial time and A is the absorbance of the sample assay after 6min of incubation. The results were expressed as Trolox Equivalent Antioxidant Capacity (TEAC) in g/g dw.
The DPPH• radical-scavenging was determined using the method proposed by Brand-Williams et al.,18 The reaction medium contained DPPH• (65μmol/L) and different concentrations of extract (3.8-25.0μg/mL). Absorbance measurements at 515nm were started immediately (t0) and after 3 h (tf) of reaction time. The results were expressed in percentage of inhibition of DPPH• according to the following equation:
DPPH• inhibition(%)=[1 – (A/ACo)]x100
Where, ACo is the absorbance of the control assay (no extract) at t0 and A is the absorbance of the sample at tf. Methanolic solutions of Trolox were tested in both ABTS•+ and DPPH• assays. The results were expressed as Trolox Equivalent Antioxidant Capacity (TEAC) in g/g dw.
Ferric reducing antioxidant power (FRAP) analysis was evaluated according to Benzie and Strain.,19 with modifications. Reaction mixtures containing 50 mL of sample (0.2g/mL) and 950mL of FRAP reagent were incubated for 30min at 37oC and the absorbances were determined at 593nm. Trolox was used as standard. The results were expressed as Trolox Equivalent Antioxidant Capacity (TEAC) in g/g dw.
Antioxidant action on O2•-, HOCl and NO•
The O2•- scavenging activity of extract was evaluated according Ewing and Janero.,20 adapted by Valentão et al.,21 and Orak et al.,22 with some modifications. Reaction medium containing PBS, NADH (166mmol/L), Nitroblue tetrazolium (43mmol/L), sample (20-160mg/mL) and phenazine methosulfate (2.7mmol/L) was incubated for 2 min at 25 °C and the absorbance was determined at 540 nm using a microplate reader (Multiskan FC Thermo Scientific, USA). The inhibition percentage of O2•- was calculated according to the following equation:
O2•- inhibition (%) = [1- (A/AC)] x 100
Where, AC is the absorbance of the control assay (no extract) and A is the absorbance of the sample. Ascorbic acid (AA) was used as standard compound.
The HOCl assay was performed according to Valentão et al.,21 with modifications. HOCl induces the oxidation of 5-thio-2-nitrobenzoic acid (TNB) to 5,5´-dithiobis (2-nitrobenzoic acid) (DTNB). The reaction was evaluated by TNB absorbance at 412nm. Reaction media containing 62.5mmol/L TNB in the absence (control) and presence of extract (100-1400mg/mL) at 25°C were used to measure absorbance at 421nm before (t0) and 5 min after (tf) the addition of HOCl. The inhibition percentage of HOCl was calculated according to the following equation:
HOCl inhibition (%)=[1- (Ai – Af)/(ACi–ACf)]x100
Where, Ai and ACi are the absorbances of the sample and control, respectively, determined before HOCl addition, and Af and ACf are the absorbance’s of the sample and control, respectively, evaluated 5min after HOCl addition. Ascorbic acid (AA) was used as standard compound.
NO scavenging activity of extract was determined according to Marcocci et al.,23 and Pooja et al.,24 based on the Griess reaction. The reaction medium contained 8.3 mmol/L sodium nitroprusside (in PBS), prepared immediately before use, in the absence (control) and presence of extract (10-200mg/mL). The mixture was incubated for 3 h at 25°C. NO2– formation was quantified by Griess reaction with sulphanilamide (58.0mmol/L) and N-(1-naphthyl)-ethylenediamine (3.86mmol/L) at 540nm using a microplate reader. The NO2– concentration was obtained using a standard curve with sodium nitrite (1.5-100mmol/L). The values were expressed as inhibition percentage of NO, after 2 h of incubation, obtained by the equation
N• inhibition (%) = [1- (A/AC)] x 100
Where, A is the absorbance of the sample and AC is the absorbance of the control. Ascorbic acid (AA) was used as standard compound.
Statistical analysis
All assays were performed in triplicate and the results represent the means±standard deviation from up to six independent extractions (n=6). The data were evaluated by analysis of variance (ANOVA), t-test and Tukey’s test for comparison of means, with a significance level of a=0.05.
Results
In present study, total phenolic contents were 0.118±0.084 g GAE/gdw. The data were mean±standard deviation (n=6).
Trolox equivalent antioxidant capacity (TEAC)
TEAC values obtained by ABTS•+, DPPH• and FRAP assays can be seen in Table 1. Results show significant difference between the values of TEAC from different methods (p<0.01), the following ascending order of antioxidant activity: ABTS•+, DPPH• and FRAP was see.
| TEAC (gTE/gdw) | ||
| FRAP | ABTS•+ | DPPH• |
| 0.282±0.023a | 0.148±0.015c | 0.215±0.076b |
Table 1 Antioxidant activities of olive leaf extract on ABTS•+, DPPH• and FRAP assays
TEAC, Trolox equivalent antioxidant capacity; TE, trolox equivalent; Mean±standard deviation (n=6). Different lowercase letters represent significant difference (p<0.01).
Antioxidant action on O2•-, HOCl and NO
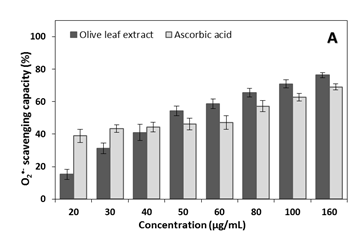
In present study, dose-response between extract concentration and inhibitory effect of reactive species was seen (Figure 1); significant differences between extract effect and ascorbic acid effect were detected. For O2•- scavenging-radical, greatest effect was observed for the extract compared to ascorbic acid, when in 50, 60, 80, 10 and 160 µg/mL; but at low concentrations (20 and 30µg/mL) ascorbic acid showed better antioxidant action than the extract (P<0.05). No difference between extract and ascorbic acid at 40µg/mL on O2•- radical-scavenging was detected. Maximum inhibition action of extract and ascorbic acid on O2•- radical-scavenging were 76.4±1.7% and 69.1±1.9%, respectively, at 160 µg/mL for both. Concentrations above 160µg/mL did not increase the O2•- radical-scavenging compared to the values found in this concentration, for both extract and ascorbic acid (results not shown).
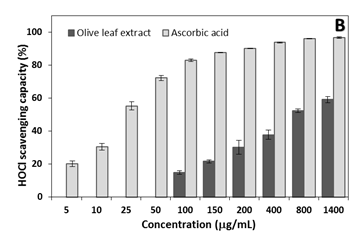
For HOCl, the extract showed inhibitory action at higher concentrations 100µg/mL, but at low concentrations (5-50µg/mL) no effect was detected. HOCl inhibition by ascorbic acid was greater than olive leaf extract in all concentration. Maximum HOCl inhibition of 96.7±0.4% and 59.1±1.8% for the respective ascorbic acid and extract at 1400µg/mL were observed; similar effects were detected at higher concentrations (above 1400µg/mL) (results not shown).
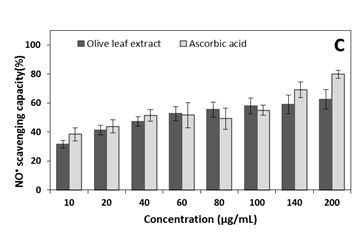
Except for 200µg/mL, no significant difference between extract and ascorbic acid on NO inhibition was detected, when ascorbic acid inhibited 80.1±4.2% and olive leaf extract inhibited 63.3±5.7% of NO. Similar inhibitions values were found at concentrations greater than 200µg /mL (results not shown).
Discussion
The antioxidant activity of olive leaf extracts against ABTS•+, DPPH• and FRAP has been verified in previous studies.6,11,21 Similar to the results presented in Table 1, olive leaf extract showed 301mg TE/g dry extract and 379.3mg TE/g dry extract for respective FRAP and ABTS•+ .24 Lins et al.,6 showed 281mg TE/g dry olive leaf extract, similar to that showed in the present work; these study found lower EC50 values (16.1µg/mL) for ABTS•+ radical-scavenging compared to EC50 (13.8µg/mL) for DPPH• radical-scavenging; suggesting higher antioxidant activity of olive leaf extract on ABTS•+ scavenging corroborating with the results of Table 1. Determination of antioxidant activity has been shown to be dependent of reaction conditions; but nevertheless studies have shown a high correlation between these different methodologies.24,25
The antioxidant capacity of olive leaves is mainly attributed to phenolic compounds presence.26–29Antioxidant action from several phenolic compounds present in olive leaves has been evaluated and antioxidant effects were related to functional groups characteristics.4,27 In addition, some studies suggest that phenolic compounds have a synergistic effect on antioxidant capacity when combined, as in olive leaf extracts.30–32 The total phenolic content found in the present study was similar to that previously found to olive leaf extract (131.7mg GAE/gdw).6 Phenolic compounds have demonstrated major role for antioxidant activity of olive leaves is attributed,26–28assessed individually or by the synergistic effect between them.26,28,30
Recent study shows a direct relation between olive leaf extract concentration and inhibition effect against O2•-, HOCl and NO,6 similarly, in the present study we observed a relation between extract concentration and inhibitory effect on reactive species (Figure 1); however, it is evident a significant differences between extract action and ascorbic acid action. Unlike the extract, ascorbic acid showed excellent antioxidant action on HOCl. Both the extract and ascorbic acid had a moderate effect on O2•- and NO scavenging, and the extract had better effect than ascorbic acid on O2•- and roughly equal values on NO scavenging. Studies show that the main polyphenols, such as oleuropein and hydroxytyrosol, have low HOCl elimination,32,33suggestive for the low antioxidant power of olive leaf extract. In fact, other studies show reduced HOCl scavenging by olive leaf extract.6 However the olive leaves extract has shown effective NO scavenger in comparison with other plants.32 Active compounds present in olive leaf such as oleuropein inhibited approximately 100% of NO formation,33 and hydroxytyrosol inhibited 61.3% of NO.34
Conclusion
Olive leaf extract showed antioxidant action on synthetic reactive species and on reactive species with biological characteristics; suggesting potentiality on biological systems. Olive leaf extract showed similarity to ascorbic acid on O2•- and NO scavenging, being less efficient over HOCl. The action mechanisms for the antioxidant effects of olive leaf extract are not fully understood. Thus, further studies will be conducted to identify compounds to elucidate the antioxidant mechanisms; In addition, further in vivo studies will be important to evaluate the antioxidant capacity of olive leaf extract.
Acknowledgments
None
Conflicts of interest
The author declares that there are no conflicts of interest
Funding
None
References
- Ghanbari R, Anwar F, Alkharfy KM, et al. Valuable nutrients and functional bioactives in different parts of olive (Olea europaea L.) – A review. Int J Mol Sci. 2012;13(3):3291–340.
- Guinda Á, Castellano JM, Santos-Lozano JM, et al. Determination of major bioactive compounds from olive leaf. LWT – Food Sci Tech. 2015;64(1):431–438.
- Lockyer S, Yaqoob P, Spencer J, et al. Olive leaf phenolics and cardiovascular risk reduction: Physiological effects and mechanisms of action. Nut Aging. 2012;1(2):125–140.
- Benavente-García O, Castillo J, Lorente J, et al. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68(4):457–462.
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352.
- Lins PG, Pugine SMP, Scatolini AM, et al. In vitroantioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon. 2018;4(9):e00805.
- Erbay Z, Icier F. The importance and potential uses of olive leaves. Food Rev Int. 2010;26(4):319–334.
- Lockyer S, Yaqoob P, Spencer JPE, et al. Olive leaf phenolics and cardiovascular risk reduction: physiological effects and mechanisms of action. Nut Aging. 2012;1(2):125–140.
- Harasym J, Oledzki RO. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition. 2014;30(5):511–517.
- Rodrigues F, Pimentel FB, Oliveira MBPP. Olive by-products: challenge application in cosmetic industry. Ind Crops Prod. 2015;70(1):116–124.
- López-Alarcón C, Denicola A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal Chim Acta. 2013;763(1):1–10.
- Floegel A, Dae-Ok K, Sang-Jin C, et al. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Comp Anal. 2011;24(7):1043–1048.
- Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53(6):1841–1856.
- Franco JM, Pugine SMP, Scatoline AM. De Melo MP. Antioxidant capacity of Melissa Officinalis L. on Biological Systems. Ecl Quím J. 2018;43(3):19–29.
- Virot M, Tomao V, Colnagui G, et al. New microwave-integrated Soxhlet extraction. An advantageous tool for the extraction of lipids from food products. J Chromat. 2007;1174(A):138–144.
- Singleton VL, Rossi Junior JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–158.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci Techn. 1995;28(1) 25–30.
- Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem.1996;239(1):70–76.
- Ewing JF, Janero DR. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem. 1995;232(2):243–248.
- Valentão P, Fernandes E, Carvalho F, et al. Antioxidative Properties of Cardoon (Cynara cardunculus L.) Infusion Against Superoxide Radical, Hydroxyl Radical, and Hypochlorous Acid. J Agric Food Chem. 2002;50(17):4989–4993.
- Orak HH, Isbilir SS, Yagar H. Determination of antioxidant properties of lyophilized olive leaf water extracts obtained from 21 different cultivars. Food Sci. Biotechnol. 2012;21(4):1065–1074.
- Marcocci L, Maguire JJ, Droy-Lefaix MT, et al. The nitric oxide-scavenging properties of ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994;201(2):748-55.
- Pooja PS, Samanta KC, Garg V. Evaluation of nitric oxide and hydrogen peroxide scavenging activity dalbergia sissoo roots. Pharmacophore. 2010;1(2):77–81.
- Hayes JE, Stepanyan V, Allen P, et al. Evaluation of the effects of selected plant-derived nutraceuticals on the quality and shelf-life stability of raw and cooked pork sausages. LWT – Food Sci Technol. 2011;44(1):164–172.
- Fernandes RPP, Trindade MA, Tonin FG, et al. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J food Sci technol. 2016;53(1):451–60.
- Goulas V, Papoti VT, Exarchou V, et al. Contribution of flavonoids to the overall radical scavenging activity of olive (Olea europaea L.) leaf polar extracts. J Agric Food Chem. 2010;58(6):3303–3308.
- Rahmanian N, Jafari SM, Wani TA. Bioactive profile, dehydration, extraction and application of the bioactive components of olive leaves. Trends in Food Sci Technol. 2015;42(2):150–172.
- Wang X, Li C, Liu Y, et al. Efficient method for screening and identification of radical scavengers in theleaves of Olea europaea L. Biomed .Chromatogr. 2011;25(3):373–380.
- Xie PJ, Huang LX, Zhang CH, et al. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure–activity relationships. J Funct Foods. 2015;16(1):460–471.
- Lee OH, Lee BY. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Biores Technol. 2010;101(10):3751–3754.
- Czerwinska M, Kiss AK, Naruszewicz M. A comparison of antioxidant activities of oleuropein and its dialdehydic derivative from olive oil, oleacein. Food Chem. 2012;131(13):940–947.
- Rietjens SJ, Bast A, Haenen GRMM. New insights into controversies on the antioxidant potential of the olive oil antioxidant hydroxytyrosol. J Agric Food Chem. 2007;55(18):609–7614.
- Conforti F, Marrelli M, Carmela C, et al. Bioactive phytonutrients (omega fatty acids, tocopherols, polyphenols), in vitro inhibition of nitric oxide production and free radical scavenging activity of non-cultivated Mediterranean vegetables. Food Chem. 2011;129(4):1413–1419.
- Ammendola S, Giusti AM, Masci A, et al. Antioxidant properties of hydroxytyrosyl acetate compared with hydroxytyrosol and their protective capacity against oxidative stress in human neuroblastoma cells. J Sci Ind Res. 2011;70(11):929–937.
 ©2020 Lins, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
©2020 Lins, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.